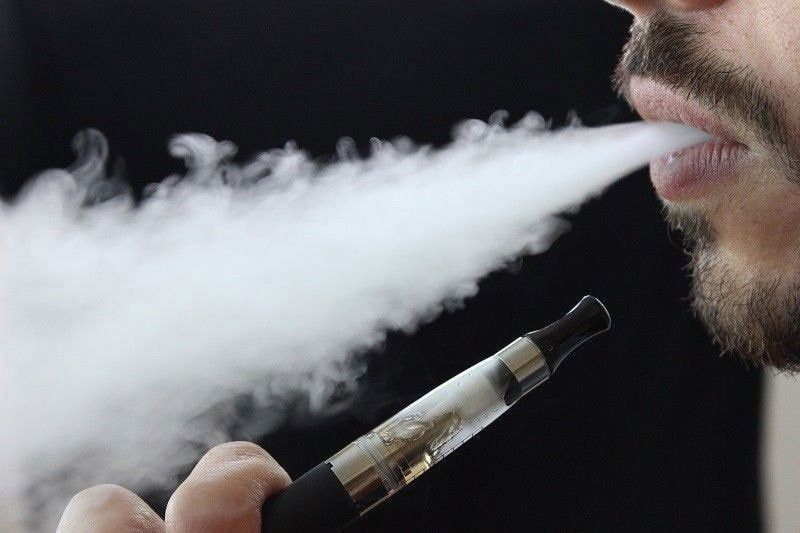
In a determined stride towards improved public health, the U.S. Food and Drug Administration (FDA) is setting its sights on electronic cigarettes (e-cigarettes). The agency’s focus is not on all e-cigarettes but specifically those that are enticing teenagers while failing to assist smokers in their cessation efforts. This blog post dives into the FDA’s initiative, the challenges it faces, and the potential implications of this action.
The FDA’s Crosshairs: E-Cigarettes That Entice Teens and Fail Smokers
The allure of e-cigarettes among teenagers has been a growing concern for years, with flavors and marketing strategies often designed to appeal to young people. Simultaneously, while some argue that e-cigarettes can help traditional smokers quit, many brands have not been able to provide substantial evidence to back these claims.
This dual problem is now the FDA’s target. The agency is stepping up its regulation and oversight efforts to curtail the availability of such products, protecting young people and promoting more effective smoking cessation tools.
Why Target E-Cigarettes?
The concern about e-cigarettes stems from their potential health risks and their increasing popularity among teens. Despite their marketed image as a safer alternative to traditional cigarettes, e-cigarettes still contain nicotine, a highly addictive substance. Moreover, the long-term health impacts of vaping are still largely unknown.
Furthermore, studies have found that teenagers who start vaping are more likely to try traditional cigarettes later, posing a significant public health issue. The FDA’s intervention aims to curb this trend and mitigate its potential consequences.
The FDA’s Action Plan
The FDA’s action plan involves rigorous evaluation of e-cigarette products, particularly those with high youth appeal or those marketed as smoking cessation aids. Manufacturers are required to demonstrate that their products provide a net benefit to public health – a high bar that not all may meet.
Implications for Public Health
The FDA’s crackdown on e-cigarettes is a significant step in promoting public health. By limiting access to products that entice teenagers and do not aid smoking cessation, the agency can help decrease the prevalence of nicotine addiction and prevent potential long-term health impacts.
However, the path forward is not without challenges. Ensuring the regulation is effective and enforced requires a diligent, multi-pronged approach and the cooperation of multiple stakeholders, including manufacturers, retailers, parents, and educators.
For an in-depth look into the e-cigarette industry and its impact on public health, visit the Campaign for Tobacco-Free Kids.
In conclusion, the FDA’s initiative to target e-cigarettes that appeal to teenagers and fail to assist smokers in quitting is a pivotal development in the fight for public health. By continuing to support such initiatives, we can hope to foster a healthier future for our youth and society at large.




































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































0