FDA Decision Raises Concerns
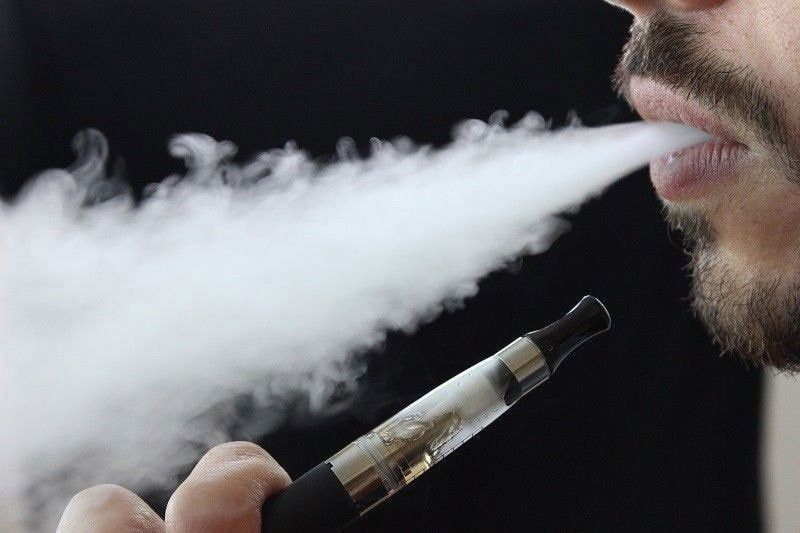
The U.S. Food and Drug Administration (FDA) recently announced its decision to deny the sale of Vuse Solo Menthol refills, citing concerns about their appeal to youth.
Addressing Youth Vaping
The FDA’s decision comes as part of its ongoing efforts to address the growing issue of youth vaping. Menthol-flavored products, in particular, have been associated with increased appeal among young individuals.
Protecting Public Health
The FDA’s primary objective is to protect public health and ensure the safety of consumers, especially vulnerable populations such as young people. By denying the sale of Vuse Solo Menthol refills, the FDA aims to reduce youth exposure to addictive substances and potential health risks associated with vaping.
Implications for the Industry
The FDA’s decision has significant implications for the vaping industry, highlighting the need for stricter regulations and measures to prevent youth access to flavored vaping products. Manufacturers and retailers will need to comply with these regulations to align with public health goals.




































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































0